Introduction: The phase 3 CARTITUDE-4 trial (NCT04181827) in patients with multiple myeloma (MM) after 1-3 lines of therapy compared ciltacabtagene autoleucel (cilta-cel) with standard of care (SOC; pomalidomide, bortezomib, and dexamethasone or daratumumab, pomalidomide, and dexamethasone). In the primary analysis, a single cilta-cel infusion significantly improved progression-free survival (hazard ratio [HR], 0.26; P<0.0001) and increased the rate and depth of response vs SOC. Here, we present adjusted comparisons of patient-reported outcomes (PROs) from patients randomized to cilta-cel vs SOC in CARTITUDE-4.
Methods:419 patients with lenalidomide-refractory MM and 1-3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory drug, were randomized (intent-to-treat [ITT] population) to receive cilta-cel (N=208) or SOC (N=211). European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30; 100-point scale), EuroQoL 5-Dimension 5-Level (EQ-5D-5L; 100-point scale), and Multiple Myeloma Symptom and Impact Questionnaire (MySIm-Q; 5-point scale) questionnaires were administered to all patients until disease progression. PRO compliance was calculated as the number of patients whose PROs were received divided by the number whose PROs were expected (ie, all patients on study pre disease progression and before subsequent therapy for each time point). Mixed-model for repeated measures analyses were performed on the ITT population to analyze changes from baseline for each arm and included the baseline PRO score and prognostic characteristics as covariates to balance arms and to adjust for confounders. Assessments after the start of subsequent therapy were excluded. Time to symptom worsening, defined as a clinically meaningful increase (≥0.5 standard deviation of pooled baseline values) without a subsequent reduction in MM symptoms, was assessed using the Kaplan-Meier method.
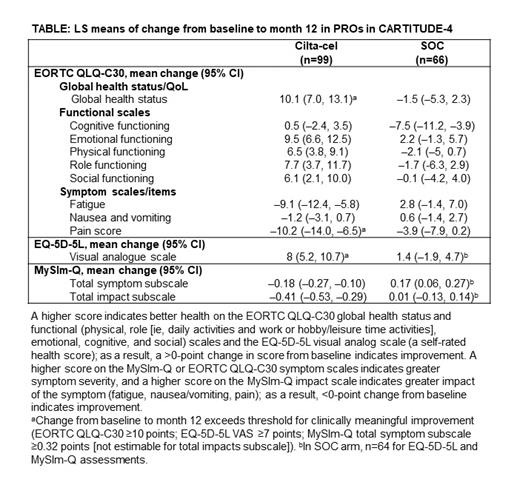
Results: At clinical cut-off on November 1, 2022, 99 patients in the cilta-cel arm and 66 in the SOC arm had both baseline and 12-month PRO assessments, representing data prior to progression. PRO compliance was 100% at baseline and decreased with subsequent visits to 74% in the cilta-cel arm and 81% in the SOC arm at month 12. Patients reported improved functioning and symptom reduction from baseline in the cilta-cel arm, while PRO scores in the SOC arm trended towards worsening or lower degrees of improvement from baseline for most domains and symptoms. The average improvement from baseline to month 12 (least squares [LS] mean change) for patients who received cilta-cel exceeded clinically meaningful thresholds for global health status (10.1 points), pain (-10.2 points), and the visual analogue scale (8.0 points); improvements in fatigue (-9.1 points) and emotional functioning (9.5 points) neared clinically meaningful thresholds (Table). For all other EORTC QLQ-C30 domains, results numerically favored cilta-cel. On the MySIm-Q total symptom scale, the median time until MM symptom worsening in the cilta-cel arm was 23.7 months (95% CI, 22.1-not estimable) and was 18.9 months (95% CI, 16.8-not estimable) in the SOC arm (HR, 0.42).
Conclusions:Patients with lenalidomide-refractory MM who had 1-3 prior lines of therapy demonstrated clinically meaningful improvements in health-related quality of life and meaningful reductions in disease-specific symptoms on multiple PRO endpoints after a single cilta-cel infusion. Improvements in health-related quality of life were numerically greater with cilta-cel than with continuously administered SOC treatments across all scales. With previously reported data showing that cilta-cel significantly improves PFS, response rate, and depth of response, these results strengthen the potential for cilta-cel to be a new SOC for patients with lenalidomide-refractory MM after first relapse.
Disclosures
Mina:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy; Pfizer: Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Yokoyama:Astellas: Honoraria. Alsdorf:Affimed: Research Funding; Biontech: Other: Travel expenses, Research Funding; GSK: Honoraria; Jannsen: Honoraria, Other: Travel expenses. Minnema:UMC Utrecht Cancer Center: Current Employment; Beigene: Research Funding, Speakers Bureau; CDR life: Consultancy; GSK: Consultancy; Janssen Cilag: Consultancy, Honoraria, Speakers Bureau. Isufi:Beam Therpauetics: Consultancy; Gilead: Consultancy, Current equity holder in publicly-traded company; Genmab: Consultancy; Abbvie: Consultancy; Incyte: Consultancy; ADC Therapeutics: Consultancy. Harrison:Haemalogix: Membership on an entity's Board of Directors or advisory committees; Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, F. Hoffmann-La Roche Ltd / Genentech, Inc., Eusa: Speakers Bureau; Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, F. Hoffmann-La Roche Ltd / Genentech, Inc., Haemalogix, Eusa, Terumo BCT: Honoraria; Celgene/BMS, GSK, Janssen Cilag, Haemalogix: Research Funding; Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, F. Hoffmann-La Roche Ltd / Genentech, Inc., Haemalogix, Eusa, Terumo BCT: Consultancy. Shah:M and M Labs: Research Funding; Sabinsa: Research Funding; C4 Therapeutics: Research Funding; Plantable: Research Funding; Bristol Myers Squibb: Consultancy, Other: Advisory Board, Research Funding; Janssen: Consultancy, Other: Advisory Board, Research Funding; Sanofi: Other: Advisory Board. Schecter:Janssen: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: Janssen. Lendvai:Janssen R&D: Current Employment, Current holder of stock options in a privately-held company. Gries:Janssen Pharmaceuticals: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Katz:Janssen Pharmaceuticals, LLC: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Slaughter:Janssen: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Lonardi:Janssen R&D: Current Employment. Deraedt:Janssen R&D: Current Employment. Costa Filho:Legend Biotech: Current Employment, Current equity holder in publicly-traded company. Patel:Legend Biotech, GSK, Freeline, BMS (spouse), AbbVie (spouse): Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Karlin:Amgen, Celgene, GSK, Janssen, Takeda: Consultancy; AbbVie, Amgen, Celgene, Janssen, Sanofi, Takeda: Honoraria. Weisel:GlaxoSmithKline: Consultancy, Honoraria, Other: Research grant to institution; Takeda: Consultancy, Honoraria, Other: Research grant; BeiGene: Consultancy, Honoraria; AstraZeneca: Honoraria; Janssen: Consultancy, Honoraria, Other: Research grant to institution; Karyopharm: Consultancy, Honoraria; Novartis: Honoraria; Oncopeptides: Consultancy, Honoraria; Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Other: Research grant to institution; Pfizer: Consultancy, Honoraria; Roche Pharma: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Other: Research grant to institution; Stemline: Honoraria; Menarini: Consultancy, Honoraria; Adaptive Biotech: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Other: Research grant to institution; AbbVie: Consultancy, Honoraria, Other: Research grant to institution.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal